Custom made tES
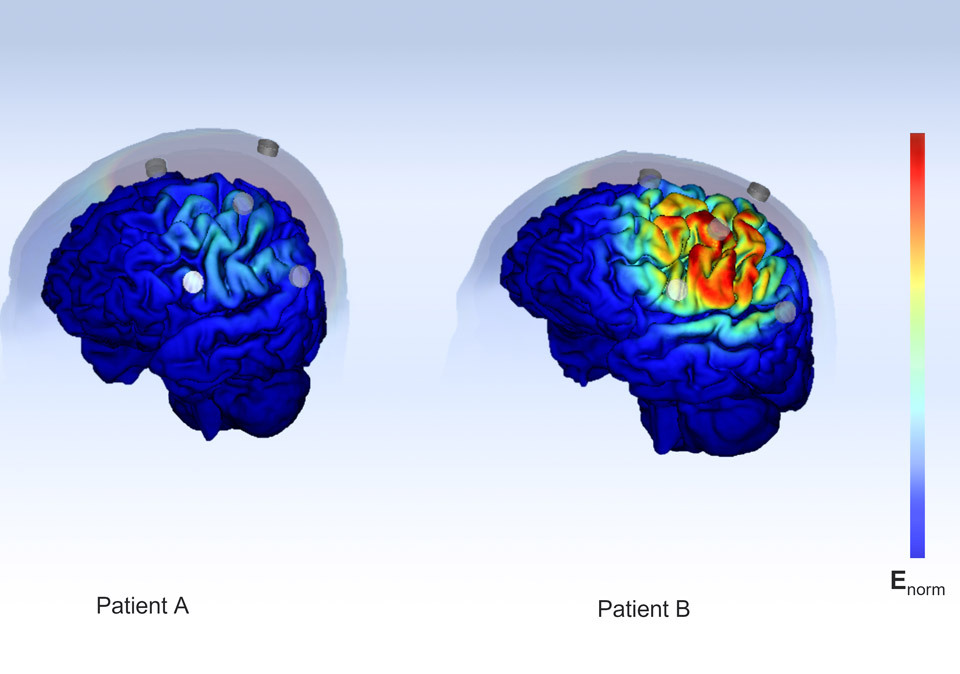
Everyone`s brain is unique. By leveraging MRI data to design therapy planning and electrode positioning, we can offer precisely targeted transcranial electrical stimulation (tES) within specific brain regions.
Our patient specific, non-invasive brain stimulation device includes a 3D printed cap fitted with up to 34 electrode channels. The cap's customized fit ensures good electrode contact that allows reproducible treatment sessions.
Our device offers both transcranial alternating current stimulation (tACS) from 20 to 80 Hz and transcranial direct current stimulation (tDCS). This multifunctionality enables us to address a wide array of neurological conditions.
The therapy plan can include multiple steps that performs EEG or stimulation patterns. For each stimulation pattern, up to 8 electrodes can be chosen and apply a combination of tACS and tDCS.
Non-invasive brain stimulation
tDCS has been shown in studies to be effective in treating depression, trauma, and stroke recovery, altering neural activity and encouraging neuroplasticity by channelling low intensity direct currents for mood stabilization, trauma healing, and rehabilitation.
tACS shows potential in managing disorders like Alzheimer’s disease, chronic pain, Parkinson’s disease and epilepsy. It modulates brain oscillatory activity, generating neural oscillations at specific frequencies in the impacted brain areas. For example, in Alzheimer's, disrupted brain oscillations and detrimentally activated microglia contribute to neuroinflammation and cognitive decline.

Treatment Procedure in 8 steps
The Bottneuro Custom tCS/EEG device is currently registered as a custom-made device (according to the UK MDR 2002 5 (1) & 15, Swiss MedDO Art. 10) for general tCS and EEG indications in the UK and Switzerland.
The prescribing qualified healthcare expert is responsible for the clinical application specific for an individual patient. The Bottneuro devices are currently not cleared for sales in the USA.